Introduction
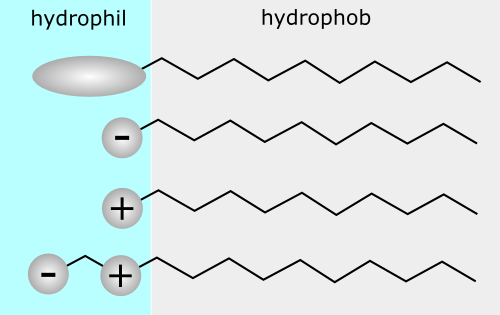
Surface active agents (usually referred to as surfactants) are amphipathic molecules that consist of a non-polar hydrophobic portion, usually a straight or branched hydrocarbon or fluorocarbon chain containing 8–18 carbon atoms, which is attached to a polar or ionic portion (hydrophilic). The hydrophilic portion can, therefore, be nonionic, ionic or zwitterionic, and accompanied by counter ions in the last two cases. The hydrocarbon chain interacts weakly with the water molecules in an aqueous environment, whereas the polar or ionic head group interacts strongly with water molecules via dipole or ion–dipole interactions. It is this strong interaction with the water molecules that renders the surfactant soluble in water.
Their primary function is to modify the interface between two or more phases in order to promote the dispersion of one phase into another. In cleaning formulations, for example, surfactants serve to wet surfaces and reduce the interfacial tension between soil and water such that the soil is removed from the surface to be cleaned and dispersed in the aqueous phase. The ability of surfactants to concentrate at interfaces derives from their amphiphilic character—the combination of hydrophilic and hydrophobic moieties within the same molecule.
Surfactants find application in almost every chemical industry, including detergents, paints, dyestuffs, cosmetics, pharmaceuticals, agrochemicals, fibres, plastics. Moreover, surfactants play a major role in the oil industry, for example in enhanced and tertiary oil recovery. They are also occasionally used for environmental protection, e.g. in oil slick dispersants.
Commercially produced surfactants are not pure chemicals, and within each chemical type there can be tremendous variation. It is advisable to obtain as much information as possible from the manufacturer about the properties of the surfactant chosen, such as its suitability for the job, its batch to batch variation, toxicity, etc. The manufacturer usually has more information on the surfactant than that printed in the data sheet, and in most cases such information is given on request.
Surfactants may be classified in several ways, depending on the needs and intentions of the people involved. One of the more common schemes relies on classification by application. Surfactants may be classified as emulsifiers, foaming agents, wetting agents, dispersants, and the like. For the user whose work is confined to one type of application, such a classification scheme has certain obvious advantages. It does not, however, tell much about the specific chemical nature of the surfactant itself, nor does it give much guidance as to other possible uses of a material.
Surfactants may also be generally classified according to some physical characteristic such as primarily water or oil solubility or stability in harsh environments.
Alternatively, some specific aspect of the chemical structure of the materials in question may serve as the primary basis for classification; an example is the type of linking group between the hydrophile and the hydrophobe (e.g., oxygen, nitrogen, amide, sulfonamido).
When discussing the commercial aspects of surfactant technology, especially with regard to the raw materials sources, it is common to refer to materials with respect to their original starting materials petroleum-based ‘‘synthetics’’ or ‘‘natural’’ oleochemicals-based materials.
A simple classification of surfactants based on the nature of the hydrophilic group is commonly used. Four main classes may be distinguished, namely anionic, cationic, amphoteric. and nonionic.
It should also be mentioned that a fifth class of surfactants, usually referred to as polymeric surfactants, has been used for many years for preparation of emulsions and suspensions and their stabilization.
Terms
Hydrophilic (‘‘water-loving’’): A descriptive term indicating a tendency on the part of a species to interact strongly with water, sometimes equated with ‘‘lipophobic,’’ defined below.
Hydrophobic (‘‘water-hating’’): The opposite of hydrophilic, having little energetically favorable interaction with water—generally indicating the same characteristics as lipophilic, except that some hydrophobic materials (e.g., perfluoro organics) can also be lipophobic.
Lipophilic (‘‘fat-loving’’): A general term used to describe materials that have a high affinity for fatty or organic solvents; essentially the opposite of hydrophilic.
Lipophobic (‘‘fat-hating’’): The opposite of lipophilic; that is, materials preferring to be in more polar or aqueous media; the major exceptions are the fluorocarbon materials, which may be both lipophobic and hydrophobic.
Lyophilic (‘‘solvent loving’’): A general term applied to a specific solute–solvent system, indicating the solubility relationship between the two. A highly water-soluble material such as acetone would be termed lyophilic in an aqueous context.
Lyophobic (‘‘solvent hating’’): The opposite of lyophilic. A hydrocarbon, for example, would be lyophobic in relation to water. If the solvent in question were changed to octane, the hydrocarbon would then become lyophilic.
Oleochemicals: Products derived from vegetable oils and similar raw materials.
Interface: The boundary between two immiscible phases (e.g., air-water interface, soil-solvent interface, fabric-water interface). Mathematically, the interface may be described as an infinitely thin line or plane separating the bulk phases at which there will be a sharp transition in properties from those of one phase to those of the other, although in fact it will consist of a region of at least one molecular thickness, but often extending over longer distances.
Interfacial tension: The property of a liquid–liquid interface exhibiting the characteristics of a thin elastic membrane acting along the interface in such a way as to reduce the total interfacial area by an apparent contraction process—thermodynamically, the interfacial excess free energy resulting from an imbalance of forces acting on molecules of each phase at or near the interface (see Surface tension).
Critical aggregation concentration (cac): A surfactant concentration at which micelle formation begins for a surfactant in the presence of polymer. The cac is an extensive characteristic of the specific surfactant–polymer system.
Critical micelle concentration (cmc): A concentration characteristic of a given surfactant at which certain solution properties change dramatically, indicating the formation of surfactant aggregates or micelles.
Dispersion: The distribution of finely divided solid particles in a liquid phase to produce a system of very high solid–liquid interfacial area.
Emulsifying agents (emulsifiers): Surfactants or other materials added in small quantities to a mixture of two immiscible liquids for the purpose of aiding in the formation and stabilization of an emulsion.
Emulsion: A colloidal suspension of one liquid in another.
Fatty acids: A general term for the group of saturated and unsaturated monobasic aliphatic carboxylic acids with hydrocarbon chains ranging from 6 to 22 carbons. The name derives from the original source of such materials, namely, animal and vegetable fats and oils.
Fatty alcohols: Primary alcohols with carbon numbers in the range of C6–C22 historically derived from natural fats and oils, directly or by reduction of the corresponding fatty acids, but more recently obtainable from petroleum sources.
Flocculation: The (often) reversible aggregation of drops or particles in which interfacial forces allow the close approach or touching of individual units, but where the separate identity of each unit is maintained.
Foam booster: An additive that increases the amount or persistence of foam produced by a surfactant system.
Foam inhibitor: An additive designed to retard or prevent the formation of foam in a surfactant solution, usually employed at low concentrations.
Hydrophile–lipophile balance (HLB): An essentially empirical method for quantifying or estimating the potential surface activity of a surfactant based on its molecular constitution—used primarily in emulsion technology.
Micelles: Aggregated units composed of a number of molecules of a surface-active material, formed as a result of the thermodynamics of the interactions between the solvent (usually water) and lyophobic (or hydrophobic) portions of the molecule.
Making Choice
The chemical structure of a surfactant is not the only determining factor in choosing between potential surfactant candidates for a given application. Economic, energetic, ecological, regulatory, and aesthetic considerations, in addition to questions of chemical functionality, are becoming more and more important in surfactant structure selection. Since most surfactants are used in formulations that include other ingredients, the relative role of the surfactant must be evaluated along with its physicochemical characteristics.
If the cost of the surfactant is significant compared to that of other components of a system, the least expensive material producing the desired effect will usually be preferred, all other things being equal. Economics, however, cannot be the only factor, since the final performance of the system will be of crucial importance.
To make a rational selection of a surfactant, without resorting to an expensive and time-consuming trial-and-error approach, the formulator must have some knowledge of:
- The surface and interfacial phenomena that must be controlled in the specific application.
- The relationships among the structural properties of the available surfactants and their effects on the pertinent interfacial phenomena to be controlled.
- The characteristic chemical and physical properties of the available surfactant choices.
- Any special chemical or biological compatibility requirements of the system.
- Any regulatory limitations on the use a given class of materials (toxicity, allergenic reactions, ecological impact, etc.)
- Public acceptance—‘‘natural’’ versus ‘‘synthetic’’ The following chapters will attempt to provide a basic foundation for making logical surfactant choices—or at least provide a good starting point and grounds for a good ‘‘educated guess.’’
References:
– An Introduction to Surfactants, Tharwat F. Tadros.
– Surfactant Science and Technology, 3rd Edition, Drew Myers.
– Handbook of Detergents, Part D: Formulation, Michael S. Showell, Procter & Gamble Company.
– Applied Surfactants, Principles and Applications, Tharwat F. Tadros.
– Surfactants, Th. F. Tadros (ed.), Academic Press, London, 1984.
– Handbook of Surfactants, M. R. Porter, Chapman and Hall, Blackie, USA, 1994.
– Surfactants and Polymers in Solution, K. Holmberg, B. Jonsson, B. Kronberg and B. Lindman, second edition, John Wiley and Sons, Ltd., Chichester, UK, 2003.