Manufacturers of cosmetics employ a surprisingly large number of raw materials. Some of these ingredients are active constituents that have purported beneficial effects on the skin, hair, or nails, for example, acting as moisturizers or conditioners. These substances are generally used in limited quantities. Other ingredients are used to formulate or create the vehicle. These are bulk chemicals used in comparatively large amounts. The resulting combination of various substances affects the nature (viscosity, oiliness, etc.) of the finished cosmetic. As a rule, numerous combinations and permutations are tested to optimize textural characteristics and to match these to consumers’ preferences. Finally, cosmetics may include substances added primarily to appeal to consumers. These ingredients need not contribute appreciably to product performance.
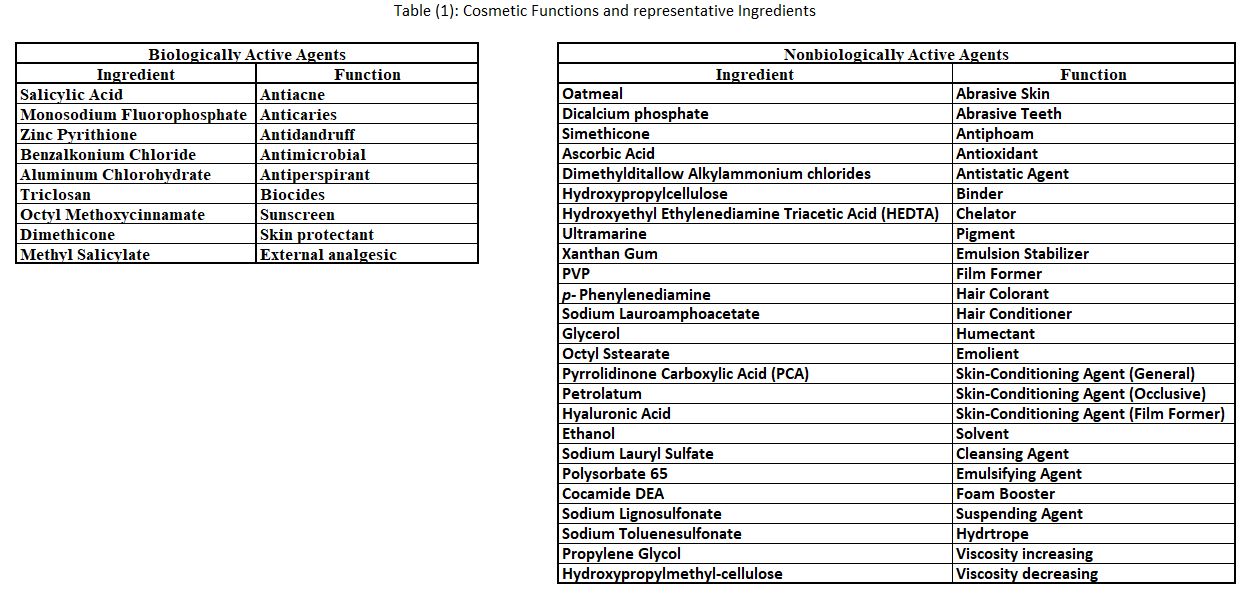
About 6000 different cosmetic ingredients have been identified. These can be divided into smaller groups according to chemical similarity or functionality. Table (1) represents a breakdown by functionality on the skin or in the product. The diversity of functions required in cosmetics is evident, and cosmetic ingredients may perform more than one function or belong to more than one chemical class.
Ingredients exhibiting certain functions are required in many types of cosmetic products. Antioxidants and preservatives are especially critical for product shelf life and quality during usage. Shelf life is defined herein as that period of time during which a product in an unopened package maintains its quality and performance and shows no physical or chemical instability.
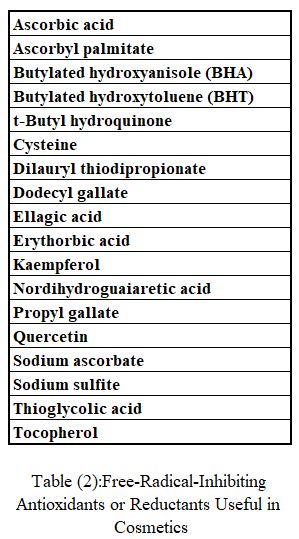
Antioxidants
Some antioxidants useful in cosmetics are listed in Table (2). The operant mechanisms are interference with radical propagation reactions, reaction with oxygen, or reduction of active oxygen species. Antioxidants are intended to protect the product but not the skin against oxidative damage resulting from ultraviolet radiation or singlet oxygen formation.
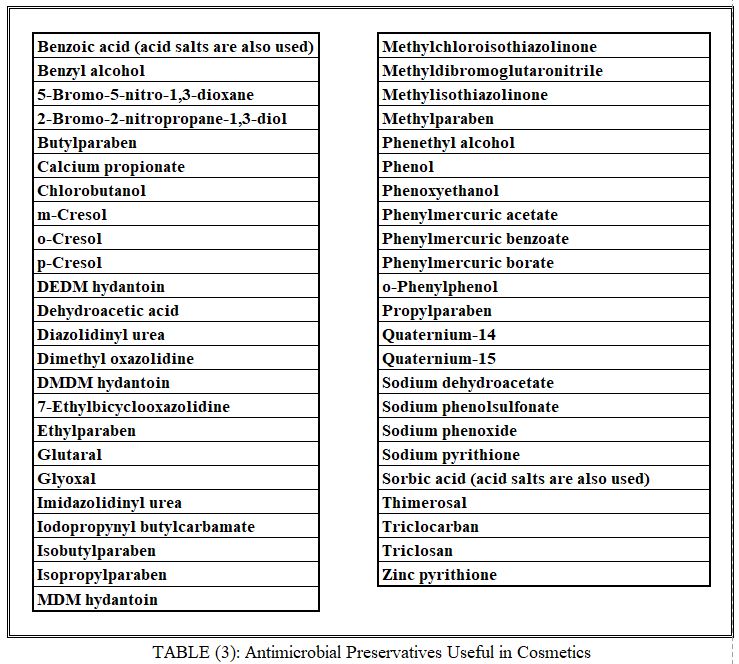
Preservatives
Several micro-organisms can survive and propagate on unpreserved cosmetic products. Preservatives are routinely added to all preparations that can support microbial growth. The choice of a preservative for a given product is difficult. Anhydrous preparations and products containing high levels of ethanol or iso-propanol may not require the addition of preservatives. Use levels are product dependent but generally do not exceed 0.25%.
Microbial integrity of products may require the presence of one or more preservatives that are compatible with the product’s ingredients. Products should not support the growth or viability of any microbial species that may have been accidentally introduced. Table 3 lists a number of antimicrobial preservatives used in cosmetic products. Experience has shown that some of the most commonly used preservatives are inactivated by a variety of surfactants. For example, the parabens (esters of p-hydroxybenzoic acid) are exceptionally sensitive to the presence of nonionic surfactants, presumably as a result of micellization of the antimicrobial by the surfactant. Over the years, preservation problems have resulted in the introduction into cosmetics of unusual substances that exhibit suitable antimicrobial spectra. However, some of these ingredients reportedly are irritants or sensitizers. Controversies in the scientific literature over the use of these substances are aggravated by regulatory acceptance or prohibition, which may differ from country to country. Table 3 includes preservatives that may be barred in certain countries. Local restrictions concerning the inclusion of preservatives and other constituents are dependent on the cosmetic product’s method of use. Products that are allowed to remain on the skin are differentiated from those that are meant to be rinsed off. Components of products left on the skin can be expected to penetrate the viable epidermis and to be systematically absorbed. Products that are rinsed off shortly after skin contact, such as shampoos, can, if properly labeled, contain preservatives that might elicit adverse reactions if left on the skin. Typical examples of such preservatives are formaldehyde, formaldehyde releasers such as Quaternium 15 or MDM hydantoin, and the blend of methylchloroisothiazolinone and methylisothiazolinone. Decorative eye cosmetic products have been reported to be subject to pathogenic microbial contamination. Regulatory agencies in several countries, therefore, permit the use of mercury-containing preservatives in eye makeups. The infections reported were to a large extent caused by contamination during use, and the introduction of self-sterilizing preparations seems warranted.
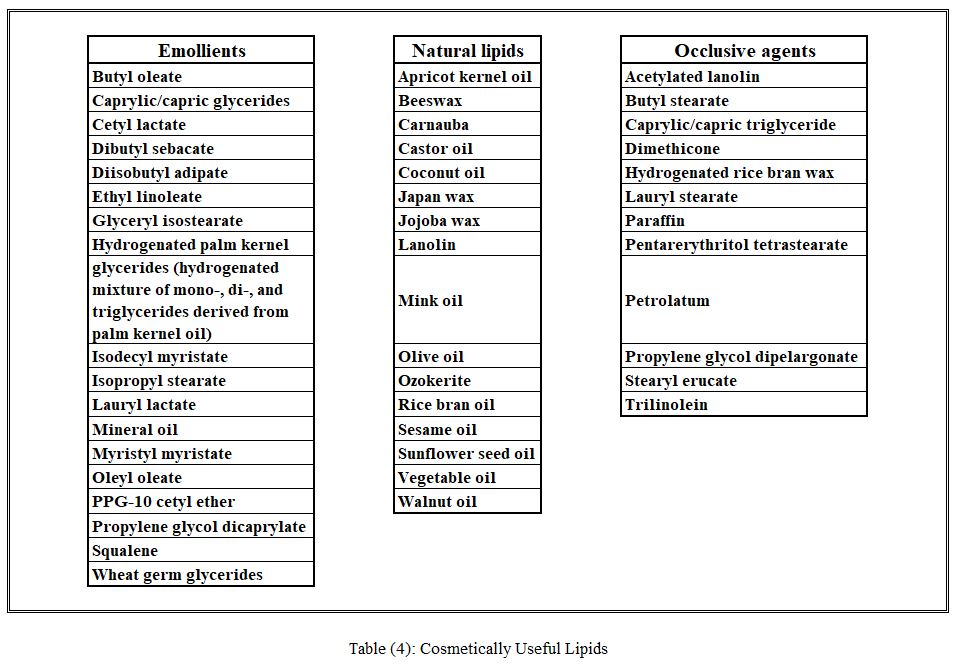
Lipids
Natural and synthetic lipids are used in almost all cosmetic products. Lipids serve as emollients or occlusive agents, lubricants, binders for creating compressed powders, adhesives to hold makeup in place, and hardeners in such products as lipsticks. In addition, lipids are used as gloss-imparting agents in hair-care products. The primary requirements for lipids in cosmetics are absence of excessive greasiness and ease of spreading on skin. Oily lipids, principal constituents of emulsions (creams and lotions), are well suited for inclusion in massage products, oils used to treat the skin (bath oils), ointments, suntan oils, and the like. Selection for a specific application is made on the basis of chemical inertness and physical properties. Petrolatum, mineral oils, polymeric silicones, polybutenes, and related substances are ingredients used for skin and hair conditioning. Conditioning is cosmetic jargon for describing a substance’s beneficial effect on the substrate. For example, quaternary compounds are substantive to skin and hair proteins and thus can produce conditioning effects. Similarly, lipidic compounds without substantive functional groups, for example, tricaprin, condition skin merely by their presence on the surface. A selected listing of cosmetically useful lipids is provided in Table 4.
Solvents
Solvents can be added to cosmetics to help dissolve components used in cosmetic preparations. Water is the most common solvent and is the continuous phase in most suspensions and water/oil (w/o) emulsions. Organic solvents are required in the preparation of colognes, hair fixatives, and nail lacquers. Selected solvents are used to remove soil, sebum, and makeup from the skin. Solvents used in cosmetics include acetone, denatured alcohol, butoxyethanol (ethylene glycol monobutylether), diethylene glycol, dimethyl isosorbide, ethyl acetate, heptane, isopropyl alcohol, mineral spirits, polyethylene glycol, propylene glycol, toluene, and tricaprin (glyceryl tri-n-decanoate).
Surfactants
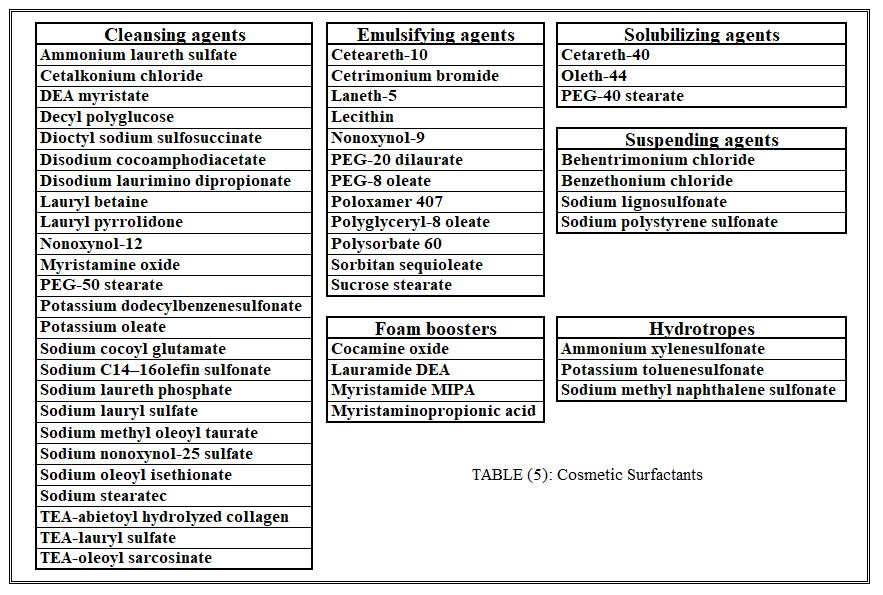
Substances commonly classified as surfactants or surface-active agents are required in a wide variety of cosmetics. These are often categorized on the basis of ionic character but are grouped in Table 5, which includes at least one member from each of the various chemical types of surfactants, on the basis of utility in cosmetics. Prolonged contact with anionic surfactants can cause some swelling of the skin. Although this is a temporary phenomenon, skin in this swollen condition allows permeation of externally applied substances. Nonionic surfactants as a group are generally believed to be mild even under exaggerated conditions. The more hydrophobic nonionics, those that are water dispersible (not water-soluble), can enhance transdermal passage. Amphoteric surfactants as a group exhibit a favorable safety profile. Finally, cationic surfactants are commonly rated as more irritating than the anionics, but the evidence for generalized conclusions is insufficient.
Colorants
Color is used in cosmetic products for several reasons: the addition of color to a product makes it more attractive and enhances consumer acceptance; tinting helps hide discoloration resulting from use of a particular ingredient or from age; and finally, decorative cosmetics owe their existence to color.
Organic Colorants
The importance of coal-tar colorants cannot be overemphasized. The cosmetic industry, in cooperation with the FDA, has spent a great deal of time and money in efforts to establish the safety of these dyes. Contamination, especially by heavy metals, and other impurities arising from the synthesis of permitted dyes are strictly controlled. Despite this effort, the number of usable organic dyes and of pigments derived from them has been drastically curtailed by regulatory action.
In addition to the U.S. certified coal-tar colorants, some noncertified naturally occurring plant and animal colorants, such as alkanet, annatto, carotene, chlorophyll, cochineal, saffron, and henna, can be used in cosmetics.
In the United States, however, natural food colors, such as beet extract or powder, turmeric, and saffron, are not allowed as cosmetic colorants. The terms FD&C, D&C, and External D&C (Ext. D&C), which are part of the name of colorants, reflect the FDA’s colorant certification. FD&C dyes may be used for foods, drugs, and cosmetics; D&C dyes are allowed in drugs and cosmetics; and Ext. D&C dyes are permitted only in topical products. Straight colorants include both the organic dyes and corresponding lakes, made by extending the colorant on a substrate such as aluminum hydroxide or barium sulfate. The pure dye content of these lakes varies from 2 to 80%; the organic dyes contain over 80% pure dye. Colorants certified for cosmetic use may not contain more than 0.002% of lead, not more than 0.0002% of arsenic, and not more than 0.003% of heavy metals other than lead and arsenic.
Inorganic Colorants
In addition to various white pigments, other inorganic colorants are used in a number of cosmetic products. These usually exhibit excellent lightfastness and are completely insoluble in solvents and water. Naturally occurring colored minerals that contain oxides of iron are known by such names as ochre, umber, sienna, etc. These show greater variation in color and tinting power than the synthetic equivalents, and the nature and amount of impurities in the national products is also variable.
Nacreous Pigments
For many years nacreous pigments were limited to guanine (from fish scales) and bismuth oxychloride. Mica, gold, copper, and silver, in flake form, can also provide some interesting glossy effects in products and on the face. Guanine is relatively costly, and bismuth oxychloride darkens on exposure to light and is difficult to suspend because of its high specific gravity. An entirely new set of colored, iridescent, inorganic pigments, which may be described as mixtures of mica and titanium dioxide (sometimes with iron oxides), has been created by coating mica flakes with titanium dioxide. The wavelengths of light reflected from these compositions can produce a complete range of colored interference patterns. The particle size of the mica must be controlled and may not exceed 150 mm, at least in the United States. Additional color effects can be created by sandwiching the mica, TiO2, and Fe2O3.
Botanicals
Plant-derived ingredients were among the first cosmetics and their use has always had a continuous interest. New discoveries of the benefits of botanicals, greater standardization and control of raw material specifications, and new formulation techniques have resulted in an explosion of interest in botanicals. Today many consumers prefer products made with natural ingredients. In the case of cosmetics, this is because good skin health is associated with natural ingredients. For this reason, essential oils extracted from plants are often added as preservatives. This new interest has resulted in the need for the industry to formulate rules for identifying the ingredients in consumer products. The earliest rules for identifying botanical ingredients for cosmetic labeling purposes were developed in the United States. Initially, it made sense to call them by their common name, e.g., apple, orange, etc. As more ingredients entered the market, it became necessary to formulate new rules. Other countries became interested in labeling botanical formulations but were worried that the names of their plant derivatives would not be understood by the rest of the world. After many meetings, The Personal Care Products Council’s (formerly the CTFA) International Nomenclature Committee recommended new rules that recognize the advantage of using scientific terminology, Latin genus, and species name as the base for botanical nomenclature. These names would be recognized by the scientific and medical community. At first in the Council’s International Cosmetic Ingredient Dictionary and Handbook (1995), the botanicals were listed as common name first and then their Latin names. In 1999, the Council considered that the familiarization of the names would be advanced enough so that they could list the names in Latin first in updated volumes of the Handbook. The Council is working to ensure that botanicals with possible health effects will continue to have their common name provided. The Council has prepared a cross-reference of Latin binomials with English common names.
Sources:
- International Cosmetics Ingredient Dictionary and Handbook, 12th ed., Personal Care Products Council, Washington, D.C., 2008.
- L. L. Schramm, Emulsions, Foams, and Suspensions: Fundamentals and Applications, Wiley-VCH, Weinheim, 2005, Chapt. 15.
- “Botanicals, Cross Reference of Latin Binomials and Common Names,” Personal Care Products Council, www.personalcarecouncil.org, accessed Jan. 2009.
- Kirk-Othmer Chemical Technology of Cosmetics. 2013 John Wiley & Sons, Inc. Published 2013 by John Wiley & Sons, Inc.













